 |
This assay will predict with high accuracy and robustness the response to Rituximab treatment and rheumatoid arthritis development and therefore is a substantial aid to the health professionals, taking the paradigm of personalized medicine one step further. |
 |
The first step: Whole blood is collected in an RNA stabilizing blood collection tube (Recommended: PAXgene blood collection tube) in which the cells are lysed, RNA is stabilized while DNA is hydrolysed. Subsequently, RNA is isolated with a blood-RNA extraction kit (Recommended: PreAnalytix Paxgene Blood RNA). |
 |
The second step: The assay will be performed from whole blood derived RNA specimen from patient which will first be turned into cDNA using the RevertAidtm H minus First-strand cDNA synthesis kit from MBI Fermentas. |
 |
The third step: the generated cDNA is placed in a pre-coated 96 wells plate. For each patient 16 candidate genes including a control gene are precoated providing space for 5 samples (10 columns) and 2 columns for 16 so-callled Calibrators (one sample could be replaced with 16 negative controls). |
 |
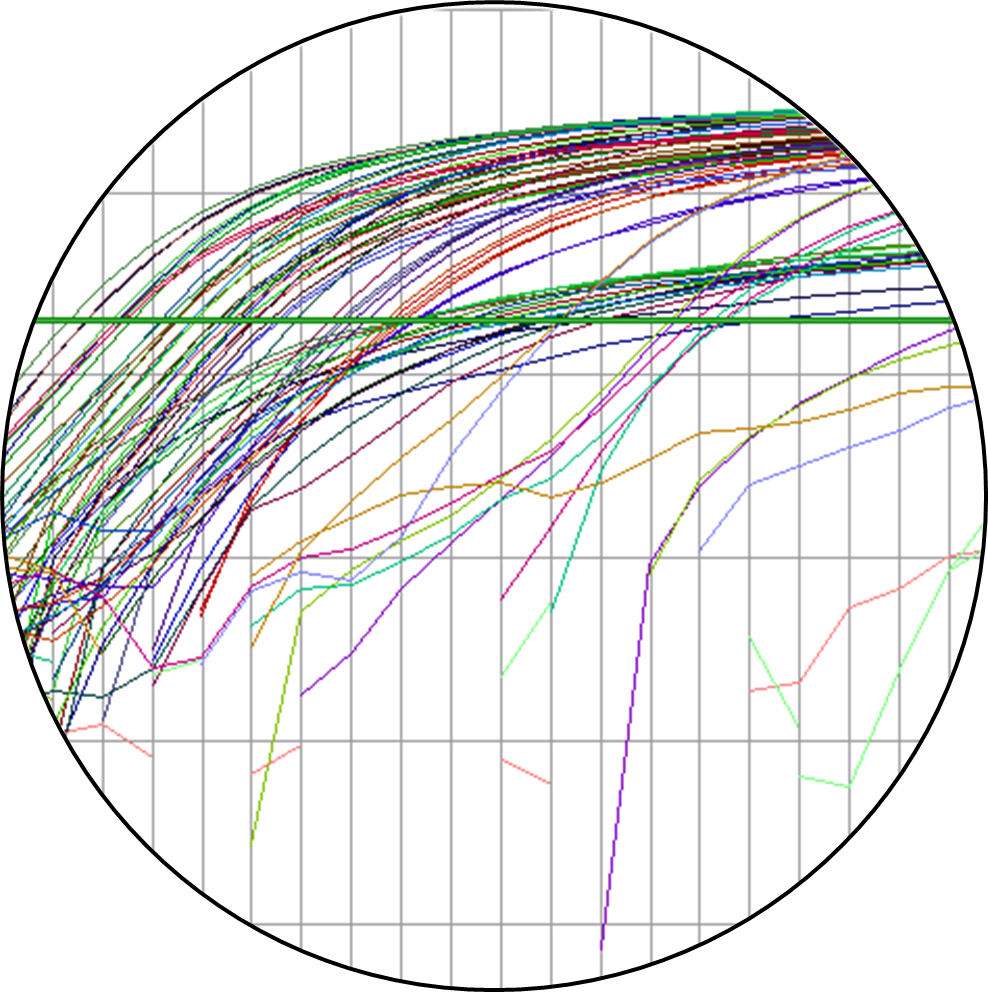
The fourth step: Quantitative real time PCR is performed using Taqman® technology for relative quantification of expression level for the 16 candidate genes. During each PCR cycle, the fluorescent signal increases in a logarithmic manner resulting in an amplification curve. As soon as the amplification curve of the target comes above a threshold, a CT value is assigned. To obtain accurate relative quantification of gene expression, an endogenous control (GAPDH) is included to normalize target quantities. |
 |
The fifth step: Eventually, quantitative results should be converted into reliable and interpretable results, providing prediction of B-cell depletion therapy response and conversion chances towards RA within 2 years, in a positive or negative outcome. For this we will collaborate with ClinicaGeno to built an on-line analyses tool based on a prediction algorithm. |
 PreselectSCANRA Therapy Response Assay
PreselectSCANRA Therapy Response Assay
 PreselectSCANRA Therapy Response Assay
PreselectSCANRA Therapy Response Assay



